Abstract
Background:
SCT400 is a recombinant, human-mouse chimeric anti-CD20 IgG1 monoclonal antibody and has the same variable regions and antigen-binding site as rituximab. SCT400 has the heavy and κ light chain constant regions of human IgG1 allotype G1m (1,17), which is common in Asians. There is only one amino acid difference between SCT400 and rituximab (V219A in the CH1 domain of the heavy chain). The phase Ⅱ study showed that pharmacokinetics, pharmacodynamics and safety of SCT400 are equivalent to rituximab in patients with CD20-positive non-Hodgkin lymphoma.
Objectives:
This phase III study aimed to evaluate the safety and efficacy of SCT400 plus cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) compared with rituximab plus CHOP in patients with treatment-naïve diffuse large B-cell lymphoma (DLBCL) (ClinicalTrials.gov, identifier: NCT02772822).
Methods:
In this multicenter, randomized, single-blind, parallel active-controlled, non-inferiority, phase III study, patients were enrolled from 37 centers in China. Eligible patients were aged 18~75 years with histologically confirmed CD20-positive, treatment-naïve DLBCL; international prognostic index (IPI) 0~2; an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0~2. Patients were randomly assigned in a 2:1 ratio to receive either SCT400 plus CHOP (S-CHOP) or rituximab plus CHOP (R-CHOP) every 21 days cycle treatment for six cycles.
The primary endpoint was objective response rate (ORR) after six cycles of therapy, assessed by an independent review committee, with a non-inferiority margin of -12%. The secondary endpoints were complete response rate (CRR), progression-free survival (PFS), overall survival (OS), and safety.
Results:
Between October 19, 2016 and July 13, 2018, 534 patients were screened and 364 eligible patients were randomized to either the S-CHOP group (n=243) or R-CHOP group (n=121). Patient demographics and disease characteristics at baseline were well balanced between the two treatment groups.
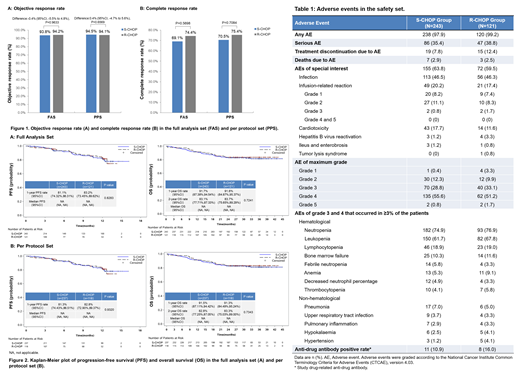
In the per protocol set (PPS), the best ORRs within six cycles of treatment were 94.5% (95% confidence interval [CI], 90.8% to 97.0%) and 94.1% (95%CI, 88.2% to 97.6%) in the S-CHOP and R-CHOP groups respectively, with an intergroup difference of 0.4% (95%CI, -4.7% to 5.6%, P=0.6569) (Figure 1A). The 95%CI lay on the positive side of the prespecified non-inferiority margin of -12%, meeting the criterion for non-inferiority. The results of using the full analysis set (FAS) were consistent with the primary efficacy analysis in the PPS. No significant differences were observed in the secondary efficacy endpoints, in either the PPS or the FAS (Figure 1B and Figure 2).
There were no significant differences were observed in ≥1 adverse event (AE, 97.9% vs. 99.2%), grade≥3 AE (85.2% vs. 86.0%), serious AE (35.4% vs. 38.8%), treatment discontinuation due to AE (7.8% vs.12.4%) between S-CHOP and R-CHOP groups, respectively (Table 1).
AEs of special interest occurred in 63.8% of patients in the S-CHOP group and in 59.5% of patients in R-CHOP group, including infection (46.5% vs. 46.3%), infusion-related reaction (20.2% vs. 17.4%), cardiotoxicity (17.7% vs. 11.6%), hepatitis B virus reactivation (1.2% vs. 3.3%), ileus and enterobrosis (1.2% vs. 0.8%), tumor lysis syndrome (0.0% vs. 0.8%) (Table 1).
A total of 51 deaths were reported in the S-CHOP group (n=35, 14.4%) and R-CHOP group (n=16, 13.2%), and 10 deaths were due to AE (S-CHOP group: n=7, 2.9%; R-CHOP group: n=3, 2.5%) (Table 1).
After treatment, study-drug related anti-drug antibodies (ADAs) were detected in 11(10.9%) patients in S-CHOP group and in 8(16.0%) patients in R-CHOP group (Table 1).
Conclusion:
SCT400 plus CHOP showed non-inferiority of efficacy to rituximab plus CHOP in patients with treatment-naïve DLBCL. The safety and immunogenicity profiles of SCT400 were comparable with rituximab, with no new treatment-related AE occurred. SCT400 could be a therapeutic option for DLBCL.
Funding source:
This study was supported by the sponsor, Sinocelltech Ltd., and by grants from the China National Major Project for New Drug Innovation (No. 2018ZX09736002 and No. 2017ZX09304015).
Conflicts of interest:
Liangzhi Xie is an employee of Sinocelltech Ltd. and has ownership interests in the company. All other authors declare no conflicts of interest.
Hu: Astellas Pharma, Inc.: Research Funding. Xie: Sinocelltech Ltd.: Current Employment, Current equity holder in publicly-traded company.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal